William Check, PhD
Numerous preanalytical and analytical factors affect the results of these assays.
Will she be able to reproduce that activity at the University of Kentucky Medical Center? And how realistic is it to think DMTs can become more commonplace?
April 2017—For decades, Michael Laposata, MD, PhD, chair of pathology at the University of Texas Medical Branch in Galveston, has touted the value of diagnostic management teams, and in February he led the first conference dedicated to such teams, referred to as DMTs. There, Alison Woodworth, PhD, told the story of how and why she created a DMT for primary hyperaldosteronism, what it achieved, and where her DMT focus is now.
“Primary hyperaldosteronism is a complex disorder that is challenging to diagnose,” she said at the conference, held in Galveston. Interpreting the screening test for primary aldosteronism, or PA, is one of the main difficulties. “We in pathology are really needed to assist clinicians in understanding what the laboratory tests mean and in understanding the limitations of laboratory tests,” said Dr. Woodworth, an associate professor of pathology and laboratory medicine and director of the core clinical laboratory and point-of-care testing at the University of Kentucky Medical Center.
A few years ago, when Dr. Woodworth was director of esoteric chemistry at Vanderbilt University Medical Center, she established a diagnostic management team for PA and evaluated its clinical utility. The DMT assisted in the diagnostic workup for PA. “We reduced the number of unnecessary tests and helped with more efficiently diagnosing the patients,” Dr. Woodworth said. Before the DMT, four of 32 patients had unnecessary testing or procedures and eight had potential delayed or missed diagnoses. After the team was implemented, there were no perceived unnecessary tests or procedures and no delayed diagnoses.
At the University of Kentucky, Dr. Woodworth is going through the process of implementing a DMT for PA in a more sophisticated format that includes implementing aldosterone and renin assays with fewer preanalytical interferences. She plans to institute a DMT for yet another challenging endocrine condition: measurement of thyroid function in pregnancy.
Dr. Woodworth embarked on her journey into the world of DMT at Vanderbilt when Dr. Laposata was Vanderbilt’s vice chair of pathology.
“When Mike came to me at Vanderbilt and said, ‘Implement a diagnostic management team in chemistry,’ I think you all can appreciate how overwhelming that seemed at the beginning,” she said at the conference.
Overwhelming, for one, because the breadth and volume of a clinical chemistry and core laboratory make it a huge task to select a condition for which to set up a DMT. “At the University of Kentucky, and probably at most of your hospitals, the clinical core laboratory is the largest laboratory by far, in terms of volume and in terms of employees,” she said. Annual test volume is about five million. “It’s a huge scope and obviously we can’t do a diagnostic management team and interpret the 20,000 laboratory test results that come into the clinical laboratory every day,” Dr. Woodworth said.
The core clinical lab serves the highest acuity patients, and rapid turnaround times are often required. It’s a complex, high-paced, fast-throughput, and automated area with a lot of volume, she said: “How do we determine just what to implement in a diagnostic management team?”
Dr. Woodworth listed three main steps in choosing which area to develop into a DMT: talk to clinical peers, choose an area with a manageable volume, and look for an application with a clinical guideline in which interpretation of laboratory data supplements results.
She began at Vanderbilt by consulting with endocrinologist Andrea Utz, MD. “At the beginning she really wasn’t sure that we in pathology could contribute to the patient care team,” Dr. Woodworth said. “It took some time and negotiation, but we did get to a point where we were able to communicate and have reasonable discussions about what might be important to implement.”

Dr. Woodworth
For test volume, “we have to have a test that’s of a manageable volume,” Dr. Woodworth said, “because we only have so many residents and fellows,” who do much of the interpretive work.
Third, “There needs to be a clinical practice guideline or some sort of evidence that we can base our interpretive reports on and not just expert opinions.”
For endocrinologists, a DMT raises concern because their job is to interpret esoteric laboratory test results. “I clarified to Dr. Utz that I wouldn’t be telling her how to interpret lab tests,” Dr. Woodworth tells CAP TODAY. “I told her I would be addressing my interpretations to an audience that has a huge volume of tests to interpret—primary care physicians and nonspecialists.” Dr. Utz eventually embraced the whole process. “She could see there could be a benefit to her service.”
Dr. Woodworth asked Dr. Utz, “How often do you get consults in which the laboratory tests were misinterpreted? How often do you see patients who are walking around out there who are not diagnosed but who have an endocrinopathy you are concerned about? Where do you most experience inappropriate lab testing—underutilization or overutilization?”
Dr. Utz named three areas that fit these criteria: thyroid disease, Cushing syndrome, and primary aldosteronism. In many cases, she said, the primary care physicians will see these patients first and they’re often confused about what tests to order and how to work up these patients. And when the results come back, they are often misinterpreted.
“The good news,” Dr. Woodworth said, “is that all three of these conditions have clinical practice guidelines that help guide the clinician’s workup for potential disease.
“I think what really drove our decision was daily test volume,” she said. “Thyroid function tests in a laboratory, particularly TSH, are about 500 a day. At Vanderbilt that was the volume, and I think that’s typical for academic medical centers of similar size. Cortisols were about 50 to 100 per day. But the screening tests for primary aldosteronism, which are plasma renin activity and aldosterone, were about 10 per day, so that was a manageable test volume that would allow our residents to identify and interpret these test results in a meaningful way.”
Setting up a DMT can be overwhelming not just because of test volume but because of the large number of people in diverse disciplines that it takes to make the team function successfully. In addition to the physicians and other medical personnel, “we also had to have people who knew the business side of things,” Dr. Woodworth said, and for this the dean’s office provided project management support. “We also had support from IT for the different aspects of programming, the EHR and/or the LIS, depending on how we wanted the results to end up in the medical record.”
Also “crucial,” she said, was a knowledge-based management resource called the Center for Knowledge Management, centered in the university library. She found clinical practice guidelines somewhat lacking in references to the primary literature. Center for Knowledge Management staff would fill that gap. “We would say, ‘Well, the guidelines say you shouldn’t measure aldosterone in the presence of this hypertensive med; please go back to the primary literature,’” Dr. Woodworth recounts. “And they would come back with a beautiful evidence-based summary that helped guide our decision-making.”
Finally, “No diagnostic management team can happen without the input of the clinical chemistry fellows and pathology residents.”
To help understand better the value of a DMT for PA, Dr. Woodworth explained the normal physiology of aldosterone and renin in blood pressure regulation and the pathophysiology of PA.
“Blood pressure is regulated in huge part by the renin-angiotensin-aldosterone system [RAAS],” she said. Low blood pressure and low sodium concentration lead to upregulation of renin from the juxtaglomerular membrane cells in the kidney, which signals conversion of angiotensinogen ultimately to angiotensin 2. Angiotensin 2 in turn acts on the adrenal glands to upregulate aldosterone secretion, which then acts on the distal tubule of the kidney to retain sodium at the expense of potassium excretion. Water follows sodium, so blood pressure goes up and sodium concentration in the blood goes up as well.
Autonomous secretion of aldosterone by the adrenal glands that is not suppressed by high blood pressure and high sodium causes dysregulation of the RAAS, which defines primary aldosteronism. “Primary aldosteronism is actually one of the more common forms of secondary hypertension,” Dr. Woodworth said. “It is the most common form of endocrine-mediated hypertension.” It has three main etiologies: aldosterone secreting tumors of the adrenal gland, bilateral adrenal hyperplasia, and, in rare cases, a familial form. Up to 15 percent of hypertensive patients may have PA.
The long-term effects of autonomous secretion of aldosterone are hypokalemia, severe hypertension, and damage to the cardiovascular system, along with sodium retention and suppressed renin activity. Because of its impact on the cardiovascular system, there have been numerous studies on how to work up primary hyperaldosteronism, Dr. Woodworth said. The Endocrine Society published practice guidelines for this workup in 2008 and updated them in 2016.
With aldosterone and renin central to this homeostatic system, it is logical that screening begins with measurement of the aldosterone-to-plasma renin activity ratio. In patients who have two abnormal screening tests, a confirmatory test is done. Typically, a confirmatory test is something that will challenge the RAAS. “The most common is a saline suppression test,” Dr. Woodworth said. “When we infuse saline, it will ultimately result in downregulation of aldosterone. However, in a patient with primary hyperaldosteronism, aldosterone will remain autonomously secreted and elevated.” But among the most important aspects of the workup is a strong screening test.
Who should undergo a screening test for PA? “The Endocrine Society practice guidelines state that we should only screen those at high risk for primary hyperaldosteronism,” Dr. Woodworth said. High-risk candidates include those with sustained high blood pressure on three different measurements over the course of several days; hypertension that is resistant to at least three hypertensive drugs; a patient who has controlled hypertension but only on four or more antihypertensives; or a patient with hypertension plus hypokalemia or hypertension plus sleep apnea. An indication of a genetic component also calls for screening, such as a person with hypertension who has a first-degree relative with primary hyperaldosteronism or a patient with hypertension with a family history of early-onset hypertension or stroke.
The aldosterone-to-plasma renin activity ratio can be measured in two ways. “Typically, in the United States, we use an aldosterone immunoassay and we measure the activity of plasma renin activity through an activity assay,” Dr. Woodworth said. In many of these cases renin activity is suppressed because of the RAAS. (Renin secretion is downregulated in the presence of high blood pressure and/or elevated sodium.) “When you have suppression of renin, the aldosterone-to-renin ratio will be high inherently,” she noted, “and so the new guidelines suggest that we shouldn’t just use the ratio of aldosterone to renin, but we should also use aldosterone concentration.” An aldosterone-to-plasma renin activity ratio, or ARR, greater than 30 with a plasma aldosterone concentration above 15 ng/dL are cutoffs most commonly cited as diagnostic for primary aldosteronism.
Most patients with PA have hypokalemia; low potassium leads to altered results for aldosterone and ARR. Sodium status is critical for maintaining the RAAS. As a result, there will be different results for plasma renin activity, aldosterone, and the ratio, depending on the patient’s diet. Posture is also a factor: Patients who are sitting upright have a higher ratio than those who are supine. And diurnal variation affects aldosterone and plasma renin activity, with both highest in the mid-morning.
Numerous medications affect the RAAS. Most prominent among them are antihypertensive medications, such as direct renin inhibitors and ACE inhibitors.
In the revised 2016 Endocrine Society practice guidelines on diagnosing PA are evidence-based recommendations for how and when to measure aldosterone and plasma renin activity. “They say you should correct the hypokalemia prior to performing these tests,” Dr. Woodworth said. “Also that you should liberalize sodium intake and discontinue many antihypertensive medications for two to four weeks. Specimens should be collected mid-morning and the patient should be awake and sitting upright for a certain amount of time because of diurnal and postural effects on aldosterone and renin.
“I’m sure that you’re sitting there thinking about how difficult it is to take patients off of these medications,” Dr. Woodworth said to the conference audience. “And that’s exactly what my endocrine partner said when I told her we had to take everybody off the medications prior to measuring these things. Sometimes it’s actually not safe for the patients. The side effects associated with going off these antihypertensives are severe, and in some cases it’s not possible. So one of the things we did was to look at how often our clinicians were measuring renin and aldosterone in an appropriate and an inappropriate way.”
Dr. Woodworth and Vanderbilt clinical chemistry fellow, Joesph Wiencek, PhD, studied 200 patients who had been worked up for PA by having aldosterone and plasma renin activity measured and the ratio calculated. “We defined suboptimal sampling conditions as samples with at least one interfering medication, those that were collected at the wrong time of day, those without known potassium status or with abnormal potassium concentration, or specimens with unknown or abnormal renal function,” Dr. Woodworth said. What they found was alarming: 85 percent of specimens were collected in a suboptimal manner. Only 15 percent were collected correctly.
“If you can’t have optimal conditions for collecting these specimens, what should you do?” Dr. Woodworth asked. The 2016 Endocrine Society practice guidelines suggest that laboratory results for aldosterone and renin be interpreted in the context of confounding factors. How can laboratorians ensure that? “The diagnostic management team,” she replied. “We were pleased with our efforts because we had implemented the diagnostic management team about two years prior to the Endocrine Society recommendations.”
Dr. Woodworth showed an example of how the team interprets results in light of the clinical history and confounding factors. “The true definition of a diagnostic management team is one that meets regularly,” she noted. “Our diagnostic management team [at Vanderbilt] met twice a week because we performed testing for renin and aldosterone twice a week. Our residents and fellows looked up clinical histories relevant to all the factors that affect test results and looked for risk factors for primary aldosteronism.”
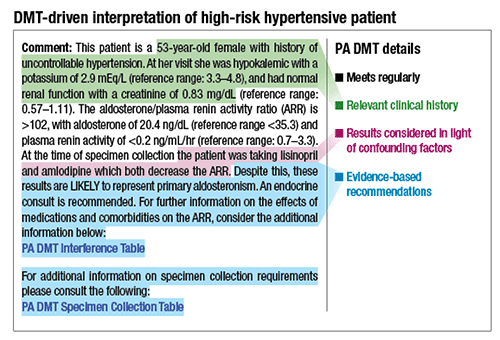
The interpretations included insight into what these test results might mean in the context of interfering medications, comorbidities, or clinical history. “And then we provided evidence-based recommendations to help the clinicians understand what the test results mean. We included risk factors, uncontrollable hypertension, hypokalemia, and then potassium and renal function status. We talked about the drugs that the patient was taking”—in this case lisinopril and amlodipine—“and we talked about how those are known to decrease the aldosterone-to-renin ratio.” (See “DMT-driven interpretation of high-risk hypertensive patient,” page 18.)
Despite that, the aldosterone-to-renin ratio was 102, and, as mentioned, the cutoff for a potential positive for primary aldosteronism is 30. “So this was probably primary aldosteronism, but because this was a screening test, we advised that the patient seek an endocrine consult for confirmatory testing.” Two links were appended: one for more information on the effects of the different drugs and comorbidities on aldosterone and renin, and the aldosterone-to-renin ratio, and another for information on collection requirements.
Dr. Woodworth and colleagues conducted a study to determine the clinical utility of the endocrine DMT. They studied four primary care practices in two periods—one year before and a little more than one year after implementing the DMT.
“Before, we had 32 patients who had been worked up for suspected primary aldosteronism,” Dr. Woodworth said, “and after we had 27. We reviewed the electronic medical records for how the patients were worked up, the diagnoses, the outcomes, and whether we were able to save unnecessary tests. We looked to see how quickly the diagnosis occurred, whether we thought the diagnosis occurred efficiently, or was delayed.”
Before implementation, four patients had unnecessary procedures, either imaging or lab tests. Potential delayed or missed diagnosis was “quite common,” Dr. Woodworth reported, “because physicians did not understand how to interpret the results of the aldosterone-to-renin ratio in the context of the medications the patients were taking. Eight patients were deemed to have either delayed or missed diagnosis.” Post-DMT, there was close adherence to the Endocrine Society practice guidelines and no perceived unnecessary testing or imaging procedures. Moreover, the five patients who were advised to have an endocrine consult did have the consults and follow-up care was appropriate.
“I got really great feedback” from clinicians about the DMT, Dr. Woodworth said. “In fact, every time Dr. Utz would get a consult as a result of the diagnostic management team, she would call me, very happy that we had provided care for the patient and that they were able to see the endocrinologist quicker.”
PhD scientists can’t bill for these consults. “Why don’t we care about billing?” Dr. Woodworth asked. The answer: Because DMT consults are good for the patient, and they are a value-added practice. “We’re providing a quicker diagnosis and fewer unnecessary tests,” she said, “so we feel like it’s a win-win for the patient.”
 “We are instituting DMTs at UK. I just presented the concept of lab utilization bundled with DMT to the CFO, CMO, and CEO. They were overwhelmingly supportive. We have support from the dean’s office and the pathology department.” Her biggest challenge will be working through necessary IT upgrades, “which was also true at Vanderbilt.”
“We are instituting DMTs at UK. I just presented the concept of lab utilization bundled with DMT to the CFO, CMO, and CEO. They were overwhelmingly supportive. We have support from the dean’s office and the pathology department.” Her biggest challenge will be working through necessary IT upgrades, “which was also true at Vanderbilt.”
One of Dr. Woodworth’s Vanderbilt colleagues, Jeremy Hart, MD, is also now at UKMC. She and Dr. Hart are on what Dr. Woodworth calls “an evangelical campaign to convert people to DMT.” They are showing data from Vanderbilt to build support among clinicians.
Dr. Woodworth will be moving one step beyond the institutional implementation of DMTs. She will propose a grant looking at how to implement DMTs. It is a targeted NIH proposal that studies the barriers to implementing processes beneficial to patients.
As for wider dissemination of DMTs, Dr. Woodworth said, “A lot of pathologists are doing something like this informally.” Dr. Laposata’s formal definition of a DMT says it must meet regularly and its interpretation must go into the patient’s chart and contain clinical information, be incorporated into the electronic health record, and be specific to that patient and relevant to patient care. “Maybe they don’t have all of these elements,” she said. “A lot of times it’s just about formalizing the process and collecting data showing its benefits.”
Can the DMT concept be extended to higher-volume tests, like the thyroid function test or workup for Cushing syndrome? Dr. Woodworth is optimistic. “In the age of informatics and big data and all the things we’re doing with next-gen sequencing, I feel like we could get to the place where we could provide personalized medicine for some of these higher-volume tests. But it would require powerful informatics tools and algorithms that we had developed ahead of time.”
The next step for the endocrine diagnostic management team is to help physicians understand the context in which they should collect the specimen and measure it. “We can’t fix all of the suboptimal conditions but we can fix many of them,” Dr. Woodworth said. She also wants to expand to other areas of endocrinology that are ripe for developing DMTs, such as thyroid function testing in pregnancy. “This is an area in which the volume would be manageable and there are lots of problems in test ordering and interpretation.”
At Vanderbilt Dr. Woodworth studied ordering patterns for thyroid function testing in pregnancy to see whether clinicians were adhering to clinical practice guidelines. “Guidelines say you should screen pregnant patients at high risk for thyroid dysfunction by measuring TSH. That wasn’t what was happening. Some practices were screening everyone,” she says. Another error was that obstetricians were measuring TSH with free T4. “So they were not screening the right population, and they were not ordering the right test. Also, when results were abnormal, they were not following up correctly.”
She did a root-cause analysis to understand the problem and found that practices doing universal screening had new obstetric patient order sets that included both TSH and T4. So the order set drove universal screening and use of the wrong test. “With regard to follow-up, they couldn’t easily see trimester-specific reference intervals in the EMR,” she said.
Building on these findings, Dr. Woodworth is working at UKMC to improve thyroid function testing ordering in pregnancy. “I am working with the endocrinology fellows here to do a similar study. We are going to put in place a diagnostic management team to mitigate the problems of misordered tests and improper workup.”
William Check is a writer in Ft. Lauderdale, Fla. The second Diagnostic Management Team Conference will take place Feb. 6–7, 2018 in Galveston, Tex.
